Potassium Hydroxide and Sulphuric Acid
Read customer reviews find best sellers. Ships Next Day Via UPS.

Write The Net Ionic Equation For The Reaction Between Sulfuric Acid And Potassium Hydroxide Homework Study Com
CH3COOH acetic acid HCOOH formic acid HF hydrofluoric acid HCN hydrocyanic acid HNO2 nitrous acid HSO4- hydrogen sulfate ion Write the balanced formula total ionic and net ionic equations for the acid base neutralization reaction that occurs when aqueous sulfuric acid is mixed with aqueous potassium hydroxide Write the balanced.

. The chemical reaction of sulfuric acid and potassium hydroxide is referred to as an acid-base neutralization. Is potassium hydroxide an acid or base. The mixture is then stirred and the highest temperature reached is 415C.
The reaction equation is H2SO4 2 KOH K2SO4 2 H2O. Ad Browse discover thousands of brands. 2 KOH H2SO4 K2SO4 2 H2O For those that suffer gout be careful with taking lots of medication for long time it can impact your health seriously.
Potassium hydroxide reacts completely with sulfuric acid according to the equation. Thermodynamic properties of substances. H2SO4 2KOH K2SO4 2H2O.
How much potassium sulfate is produced from 1075 g of sulfuric acid. In these types of reactions the acid and base mix together to form water and a salt although not necessarily table salt. Write the balanced NET ionic equation between sodium phosphate and magnesium nitrate.
Acid metal hydroxide â salt water. Likewise the K will stick to the SO42- to make Na2SO4 salt. Sulfuric acid and potassium hydroxide produce the salt potassium sulfate.
Sulphuric acid and potassium hydroxide yield potassium. Write molecular ionic and net equations for the following acid base neutralization. Drugs just treat the symptoms not the disease.
Typical neutralization reactions are. Therefore sulphuric acid is not used during the reaction of alcohols with KI. Hence in the given reaction potassium hydroxide and sulphuric acid are the reactants and potassium sulphate and water are the products.
The chief purpose of the present paper is to provide convenient access to up-to-date. Write a balanced chemical equation for the acid-base reaction of oxalic acid H2C2O4. What do we get on reacting potassium hydroxide with sulphur If you leave a concentrated solution of potassium hydroxide in contact with powder sulfur for a long time days in a sealed recipient you will see the solution turn yellow and the sulfur will dissolve.
When solutions of magnesium sul ate and calcium chloride are mixed calcium sulfate precipitates. To demonstrate this I. KOH HCl KCl H 2 O.
Suggest Corrections 0 Similar questions Q. The complete ionic equation of the reaction is Slides 11-12. The neutralization reaction of sulfuric acid with a base is highly exothermic in some cases the solution can and will boil violently.
In an experiment to determine the heat of neutralisation 50 cm 3 of 10 mol dm -3 sulphuric acid at 285C is added to 50 cm 3 of 20 mol dm -3 potassium hydroxide solution which is also at 285C in a plastic cup with a cover. Make sure formulas of all reactants and products are correct before balancing the equation. Potassium hydroxide reacts with sulfuric acid.
Complete the reaction between potassium hydroxide aq andsulfuric acid aq. In this case our acid is sulfuric acid H2SO4 and our base is potassium hydroxide KOH. In this case our acid is sulfuric acid H2SO4 and our base is potassium hydroxide KOH.
2 Na3PO4 3 Mg NO32 ----------- 6 NaNO3 Mg3 PO42. Potassium hydroxide and sulfuric acid ionic equation General word equation. Calculate the heat of neutralisation.
What are the products of the reaction between Bromic acid and. H 2 SO 4 aq 2KOHaq K 2 SO 4 aq 2H 2 Ol. Likewise the K will stick to the SO42- to make Na2SO4 salt.
The result is a complex solution of potassium polyssulfides. Acid-Base Reactions a. CsOH Cesium hydroxide nitric acid hydrofluoric acid barium hydroxide nitrous acid sulfuric acid acetic acid hydrogen sulfide potassium hydroxide hydroiodic acid perchloric acid lactic acidHC 3H 5O 3 hypochlorous acid ammonia 3 Learning check.
Hence the correct word equation of the given reaction can be written as Potassium hydroxide Sulphuric acid Potassium sulphate Water The correct answer is C. Ad We Offer a Wide Variety of Potassium Hydroxide Products. The frequency of references to these papers testifies to the important part they have played in making the methods available to biologists.
Periodic table of elements. Potassium hydroxide reacts with sulfuric acid. Equations of Aqueous Reactions a K aq OH.
Potassium hydroxide is an. Specializing in the Development and Manufacturing of High Quality Chemicals. As a result the reaction between alcohol and HI to produce alkyl iodide cannot occur.
Check the balance Sulfuric acid react with potassium hydroxide to produce potassium sulfate and water. 500ml 1 Liter and 4 Liter Sizes. The solubility of the substances.
25 cm 3 samples of this solution were titrated against sulfuric acid H 2 SO 4 solutionThe mean volume titre of sulfuric acid required for. Write the complete balanced for the reaction between potassium bicarbonate and phosphoric acid. Now this is also a double replacement reaction the H from the H2SO4 will attach to the OH- from the KOH to make H2O water.
Now this is also a double replacement reaction the H from the H2SO4 will attach to the OH- from the KOH to make H2O water. Potassium Hydroxide is a strong base in terms of chemical ionization and solutions of it can be assayed using a strong acid such as Hydrochloric Acid or Sulfuric Acid or by using an acidimetric standard such as Potassium Hydrogen Phthalate KHP. By means of sulphuric acid or potassium hydroxide solutions has been increasingly widely practised.
The coffecient sulfuric acid in the balanced equation with smallest whole number sufficients is Slide 22. Potassium hydroxide KOH and sulfuric acid H2SO4 react to make potassium sulfate and water. Buy Sulfuric Acid Products 20 Concentrated 98 H2SO4 For Gold Recovery And Refining.
095 g of potassium hydroxide were dissolved in water and made up to 250 cm 3 of solution. Potassium sulfate and water are formed in the reaction. Sulfuric acid - diluted solution.
Ad Sulfuric Acid For Sale Online. H 2 SO 4 2KOH K 2 SO 4 2H 2 O. What is the reaction between Sulphuric acid and potassium hydroxide.

Type Of Reaction For Koh H2so4 K2so4 H2o Youtube

Balance Koh H2so4 K2so4 H2o Potassium Hydroxide And Sulfuric Acid Youtube

Balance Koh H2so4 K2so4 H2o Potassium Hydroxide And Sulfuric Acid Youtube
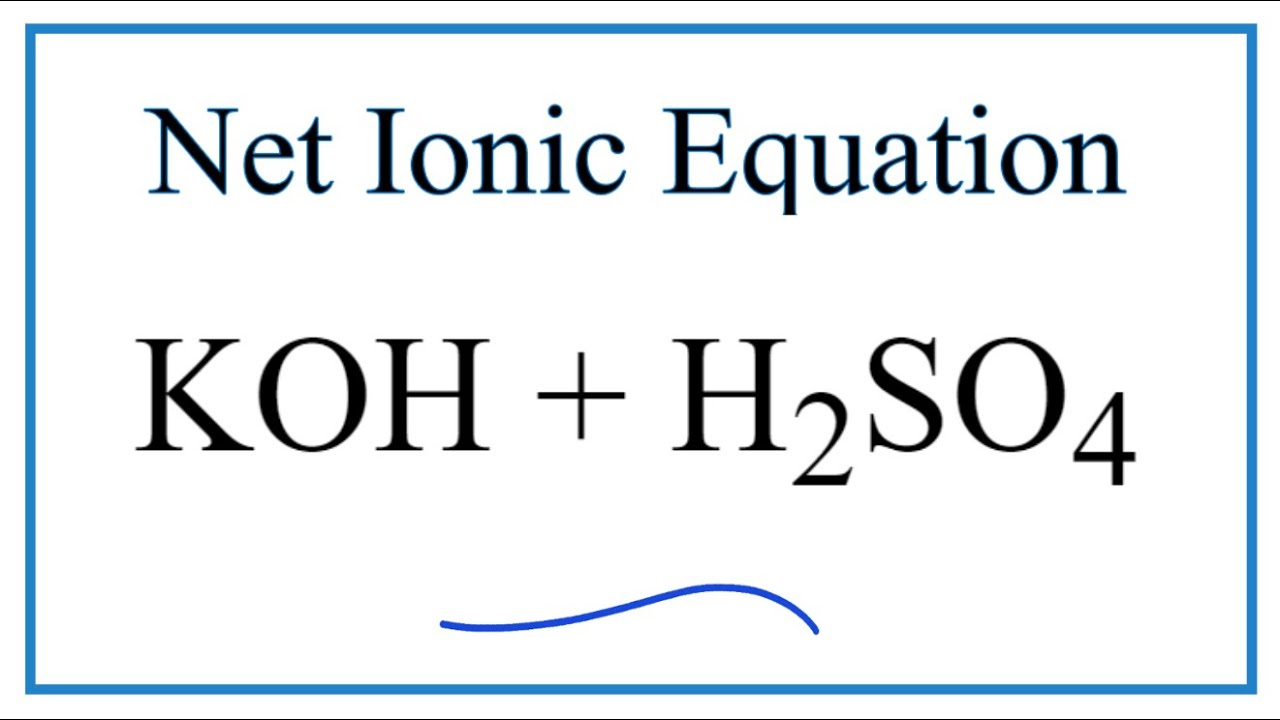
How To Write The Net Ionic Equation For Koh H2so4 K2so4 H2o Youtube
No comments for "Potassium Hydroxide and Sulphuric Acid"
Post a Comment